At Ambu we value independent research because we believe it can advance medical and scientific knowledge and ultimately improve patient care. On this page, you can learn about Ambu Investigator Initiated Studies (IIS) and apply for support.
Contact us with your proposal
Ambu supports Investigator Initiated Research Projects wherever feasible across the globe. If you would like to submit a project, please fill in our application form.
Learn more about Ambu IIS projects
If you are not ready to submit an application, you can reach out with your questions or review the FAQ below.
Frequently Asked Questions
Investigator Initiated Research Projects
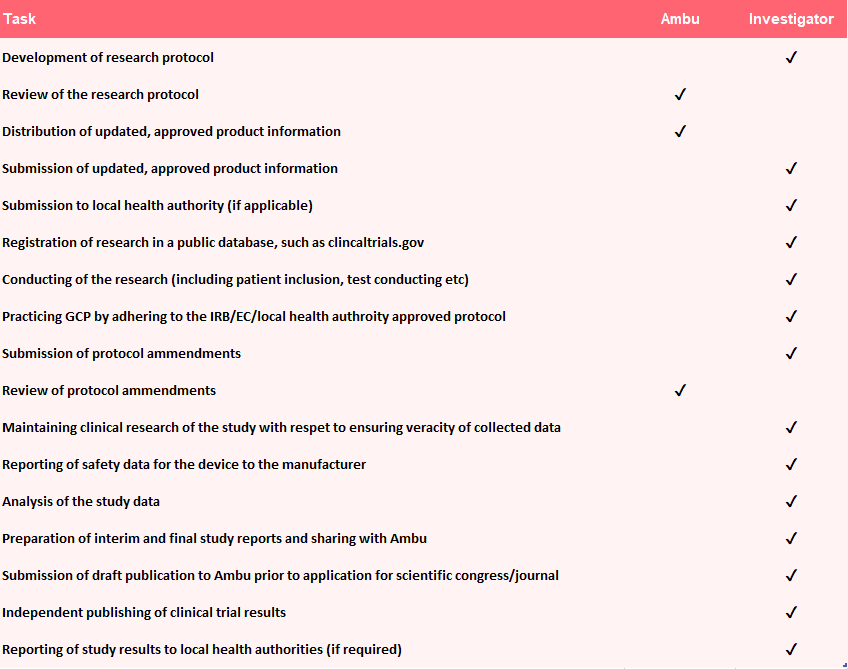
What will Ambu’s role be in your Investigator Initiated Studies (IIS)?
Research is conceptualised, initiated, and conducted by an investigator who has full responsibility over the research program. The investigator or institutions that employ the investigator maintains sponsor responsibility for the study. It is never Ambu.
Contractual responsibilities will be defined explicitly in an IIS agreement prior to the start of the study.
Below in an overview of how expectations could be aligned.
Who can apply to be an investigator?
Any healthcare professional involved in clinical research, including PhD and postdoctoral students, are invited to submit an application. While experienced researchers and clinicians who have completed previous studies are encouraged to apply, we also value and wish to support promising new clinicians and researchers.
What type of support could be funded via Ambu’s IIS program?
While there is no comprehensive list, these are some examples of the support we might offer:
- medical device products
- equipment/supplies
- data management software/data management tools
- study-related personnel costs (this could include a statistical support or research personnel)
How do I submit my application for an IIS?
Mandatory information includes, but is not limited to:
- details of the sponsoring investigator or institution
- overall objectives and outcome to be measured in the research (including the proposed evaluation methods)
- information on the study design and anticipated duration
- type of support/funding requested
- relevant budget details
- synopsis/summary of the protocol
- all support by Ambu must be declared in publication
What type of research is of interest to Ambu?
We encourage research studies that aim to improve the overall quality of patient care by exploring clinical and health outcomes.
Please note that the studies must include one or more of Ambu’s products and be aligned with our areas of strategic interest.
Current areas of interest include:
- procedures with aScope 5 Broncho HD
- difficult airway management with VivaSight 2 SLT
- anaesthesia and airway management
When can I expect a response?
Timelines for application assessment and approval can vary. You can expect a response to your initial enquiry or application within 30 days.
How will my proposal be evaluated?
Support is awarded based on the study’s scientific merit and research priorities, current scientific trends and available resources.
Once my project has been approved, what documents will I need prior to commencing my study?
Following approval, Ambu will need to receive the following in order to issue the requested support:
- a fully executed IIS contract
- institutional Review Board IRB or ethics committee Letter of Approval/Letter of Exemption if applicable
Is it necessary to register my clinical study on a clinical trials website?
Yes. It is the investigator/institution's responsibility to report any clinical research on humans to a clinical trials website such as ClinicalTrials.gov.
Who is responsible for reporting any study-related adverse events?
It is the investigator’s responsibility to report any study-related adverse events as required according to national and institutional reporting procedures.
I would like to know more about Investigator Initiated Studies – Please contact me
Apply for Ambu Investigator Initiated Study Support
In order to increase your chance for a successful application, please complete as much of this form as possible. Applications are reviewed throughout the year when the IIS committee meets. You can expect a response within 30 days. If you are not ready to fill in the form, please review the FAQ or contact us with your questions.


